C2C12
CRL-1772 ™
EXPLORE OUR DATA
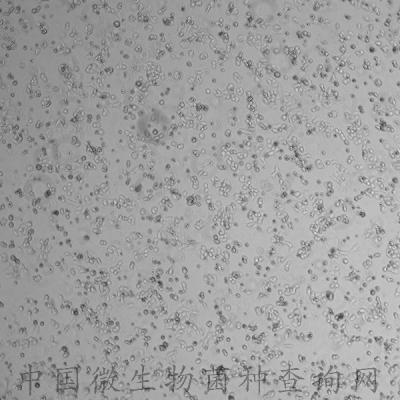
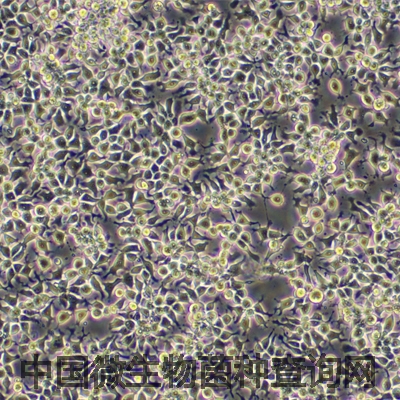
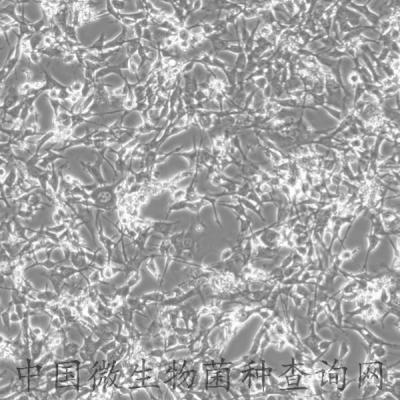
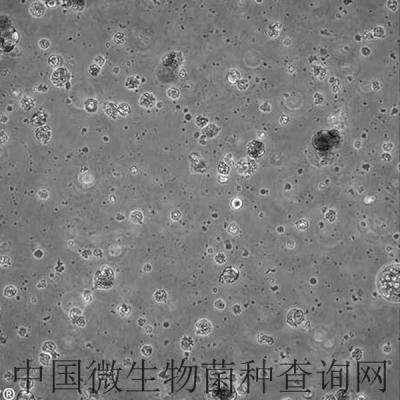
C2C12 is a myoblast cell line that is a subclone (produced by H. Blau, et al) of the mouse myoblast cell line established by D. Yaffe and O. Saxel. The C2C12 cell line differentiates rapidly, forming contractile myotubes and producing characteristic muscle proteins. Treatment with bone morphogenic protein 2 (BMP-2) cause a shift in the differentiation pathway from myoblastic to osteoblastic.
Product category
Animal cells
Organism
Mus musculus, mouse
Classification
Eukaryota, Animalia, Metazoa, Chordata, Vertebrata, Tetrapod
Cell type
myoblast
Morphology
myoblast
Tissue
Muscle
Applications
3D cell culture
Food production research
Product format
Frozen
Storage conditions
Vapor phase of liquid nitrogen
General
Specific applications
transfection host
Potential cell line for cultivated meat production
Characteristics
Growth properties
Adherent
Derivation
This is a subclone (produced by H. Blau, et al) of the mouse myoblast cell line established by D. Yaffe and O. Saxel.
Strain
C3H
Comments
The C2C12 cell line differentiates rapidly, forming contractile myotubes and producing characteristic muscle proteins.
Treatment with bone morphogenic protein 2 (BMP-2) cause a shift in the differentiation pathway from myoblastic to osteoblastic.
Tested and found negative for ectromelia virus (mousepox).
Handling information
Unpacking and storage instructions
Check all containers for leakage or breakage.
Remove the frozen cells from the dry ice packaging and immediately place the cells at a temperature below -130°C, preferably in liquid nitrogen vapor, until ready for use.
Complete medium
The base medium for this cell line is ATCC-formulated Dulbecco's Modified Eagle's Medium (ATCC 30-2002). To make the complete growth medium, add the following components to the base medium: fetal bovine serum (ATCC 30-2020) to a final concentration of 10%.
Temperature
37°C
Seeding density
5.0 x 103 viable cells/cm2
Handling procedure
Handling Procedure for Frozen Cells
To insure the highest level of viability, thaw the vial and initiate the culture as soon as possible upon receipt. If upon arrival, continued storage of the frozen culture is necessary, it should be stored in liquid nitrogen vapor phase and not at -70°C. Storage at -70°C will result in loss of viability.
SAFETY PRECAUTION: ATCC highly recommends that protective gloves and clothing always be used and a full face mask always be worn when handling frozen vials. It is important to note that some vials leak when submersed in liquid nitrogen and will slowly fill with liquid nitrogen. Upon thawing, the conversion of the liquid nitrogen back to its gas phase may result in the vessel exploding or blowing off its cap with dangerous force creating flying debris.
Thaw the vial by gentle agitation in a 37°C water bath. To reduce the possibility of contamination, keep the O-ring and cap out of the water. Thawing should be rapid (approximately 2 minutes).
Remove the vial from the water bath as soon as the contents are thawed, and decontaminate by dipping in or spraying with 70% ethanol. All of the operations from this point on should be carried out under strict aseptic conditions.
Transfer the vial contents to a centrifuge tube containing 9.0 ml complete culture medium. and spin at approximately 150 to 400 x g for 8 to 12 minutes.
Discard the supernatant and resuspend the cell pellet in an appropriate amount of fresh growth medium.
Transfer the cell suspension to an appropriate size vessel. It is important to avoid excessive alkalinity of the medium during recovery of the cells. It is suggested that, prior to the addition of the vial contents, the culture vessel containing the growth medium be placed into the incubator for at least 15 minutes to allow the medium to reach its normal pH (7.0 to 7.6).
Incubate the culture at 37°C in a suitable incubator. A 5% CO2 in air atmosphere is recommended if using the medium described on this product sheet.
Subculturing procedure
IMPORTANT - DO NOT ALLOW CULTURES TO BECOME CONFLUENT.
Cultures must not be allowed to become confluent as this will deplete the myoblastic population in the culture.
Myotube formation is enhanced when the medium is supplemented with 10% horse serum instead of fetal bovine serum.
Remove and discard culture medium.
Briefly rinse the cell layer with D-PBS (ATCC 30-2200) to remove all traces of serum which contains trypsin inhibitor.
Add 2.0 to 3.0 mL of Trypsin-EDTA solution (ATCC 30-2101) to flask and observe cells under an inverted microscope until cell layer is dispersed (usually within 5 to 15 minutes).
Note: To avoid clumping do not agitate the cells by hitting or shaking the flask while waiting for the cells to detach. Cells that are difficult to detach may be placed at 37°C to facilitate dispersal.
Add 6.0 to 8.0 mL of complete growth medium and aspirate cells by gently pipetting.
Add appropriate aliquots of the cell suspension to new culture vessels.
Inoculate at a cell concentration between 1.5 X 10 5 and 1.0 X 10 6 viable cells/75 cm2. Corning T-75 flasks (catalog #430641) are recommended for subculturing this product.
Incubate cultures at 37°C.
Medium Renewal: Every two to three days
Reagents for cryopreservation
Complete growth medium supplemented with 5% (v/v) DMSO (ATCC 4-X)
Quality control specifications
History
Legal disclaimers